Understanding the Voltage Clamp: A Practical Guide for New Researchers
The voltage clamp technique lets you hold a membrane at a precise potential while you measure the ionic current required to keep it there. By fixing voltage and watching current, you can isolate the behavior of ion channels and transporters with clarity—an approach that underpins epithelial transport studies (e.g., CFTR), Ussing chamber workflows, neuronal and cardiac electrophysiology, and ion-channel pharmacology.

👉What Is a Voltage Clamp?
A voltage clamp clamps (holds) the membrane potential at a chosen “command voltage.” If channels open or close, the membrane would normally drift—so the clamp injects just the right amount of current to counter that drift and keep voltage steady. The injected current is your data: it mirrors the ionic currents flowing through the tissue or cell.
Think of it like water pressure: you hold the pressure constant (voltage) and measure how much water you must add or remove (current) to keep it steady. Those adjustments reveal what the pipes and valves (ion channels) are doing inside.
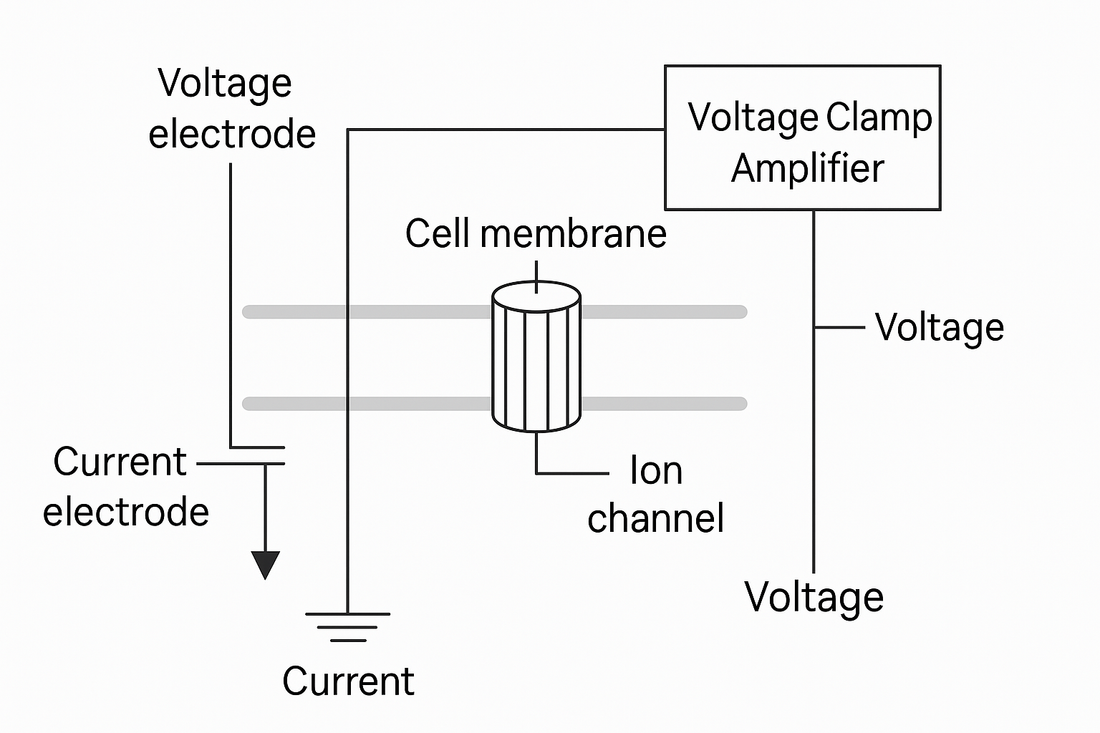
📢 Core Components (and What Each One Does)
- Measuring (voltage-sensing) electrodes — read the instantaneous membrane potential.
- Current-passing electrodes — deliver just enough current to bring the membrane back to the set point.
- Voltage clamp amplifier — compares measured voltage to the command voltage and drives a feedback response.
- Feedback loop — a fast control system that continuously injects current to eliminate any voltage error.
In Ussing chamber work, these functions are provided by integrated systems such as the VCC MC8 voltage/current clamp amplifier, working with a tissue mounted in a chamber like the EasyMount Ussing Chamber (P2300).

🔔 How the Voltage Clamp Actually Works (Step by Step)
- Choose a command voltage (Vcmd). Example: 0 mV for standard short-circuit conditions in epithelia.
- Measure the membrane potential (Vm). Sensing electrodes read Vm continuously.
- Compare and correct. The amplifier computes the error (Verr = Vcmd − Vm) and injects current to reduce Verr to ~0.
- Record the injected current (I). That current equals the net ionic current crossing the tissue or membrane at the clamped voltage.
Because voltage is fixed, changes in the recorded current directly report channel/transporter activity. Add a drug, switch the perfusate, or open CFTR—your current trace shows the effect in real time.
⚙️ Voltage Clamp Technique (Detailed Step-by-Step)
The voltage clamp technique is the cornerstone of quantitative electrophysiology. Below is a detailed walkthrough of how researchers implement this method in practice:

- Prepare the system. Mount your tissue or cell layer in the Ussing chamber, ensuring both sides are perfused with identical electrolyte solutions.
- Insert electrodes. Place paired voltage-sensing and current-passing electrodes symmetrically on each side of the tissue. Confirm clean, bubble-free connections.
- Zero the baseline. With identical solutions, adjust offsets on the amplifier until the measured potential is 0 mV.
- Apply the command voltage. Set your target membrane potential (e.g., 0 mV or a chosen step voltage). The amplifier now maintains this potential automatically.
- Record ionic currents. Observe how the injected current changes as ions move across the tissue. Each deflection represents a physiological transport event.
- Introduce stimuli. Add pharmacological agents or modify solutions to reveal specific channel or transporter functions (e.g., block ENaC or activate CFTR).
- Analyze the trace. Export data into Acquire & Analyze for quantification, normalization to surface area, and annotation of each experimental step.
This expanded sequence emphasizes precision and reproducibility—the two most critical aspects of voltage clamp methodology. Each adjustment made by the amplifier represents a direct electrical mirror of the underlying ion transport.
📌 Why Clamp? Advantages for Epithelial & CFTR Research
- Dissect specific pathways. Hold voltage constant to isolate ion currents (e.g., chloride via CFTR) without voltage-driven confounds.
- Quantify transport under defined conditions. Pair clamped recordings with perfusate changes to build clean stimulus–response relationships.
- Enable pharmacology. Resolve agonist, inhibitor, and modulator effects with precise timing and amplitude.
For a complete workflow, combine the P2300 chamber with the VCC MC8 and Acquire & Analyze software for streamlined acquisition, annotation, and export.