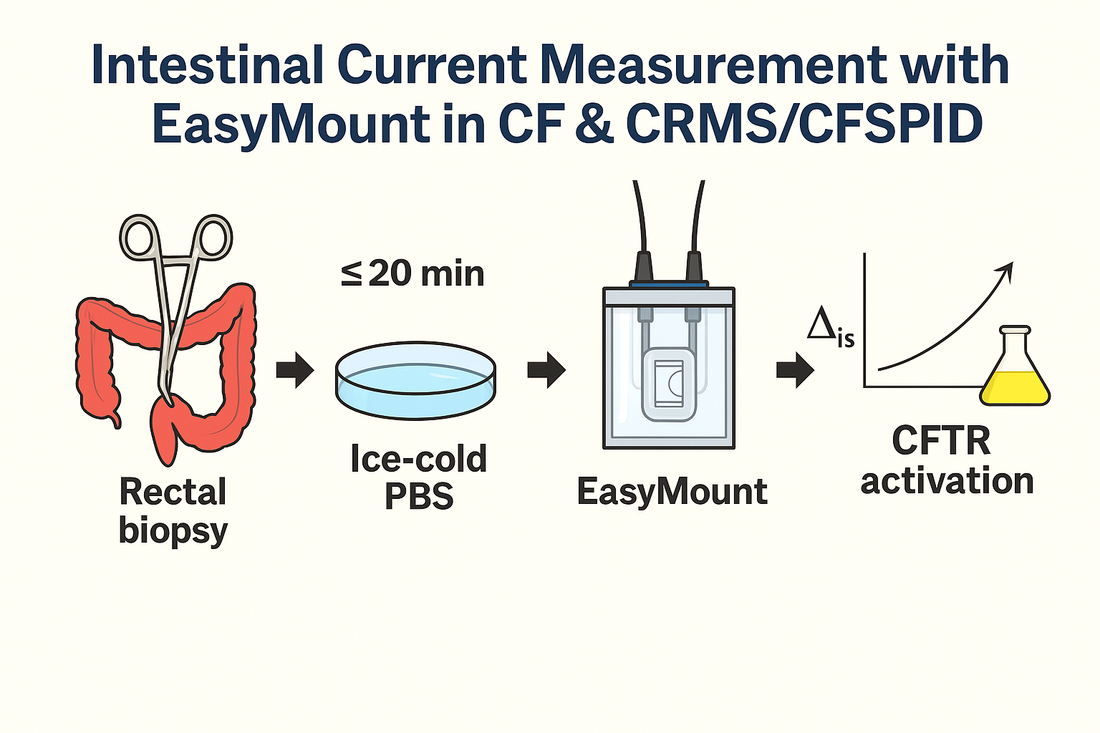
A new Polish pilot study shows how to run intestinal current measurement (ICM) in rectal biopsies using the EasyMount® Low-Volume Ussing System P2400 and P2407B slider, offering a blueprint PIs can follow to generate high-fidelity CFTR readouts across CF, CRMS/CFSPID, and healthy cohorts. Link to article: https://www.mdpi.com/2077-0383/14/17/6020
Key takeaways (for lab leads):
-
The authors implemented ECFS DNWG SOP v2.7 and explicitly reported the EasyMount P2400 + P2407B hardware—excellent for method reproducibility and equipment transparency.
-
Their workflow pinned down tight handling windows: 10–20 min from biopsy to mounting; pre-incubation at 37 °C, viability threshold Rt > 10 Ω·cm²; and a defined stimulant sequence (amiloride → IBMX/forskolin → carbachol → genistein → DIDS → histamine).
-
Cumulative ΔIsc after carbachol + IBMX/forskolin + histamine clearly separated CF from CRMS/CFSPID and healthy controls, supporting ICM’s diagnostic value when sweat chloride is equivocal.

Why this matters now - ICM
ICM is a sensitive ex-vivo bioassay of CFTR function and increasingly complements sweat testing and NPD when the diagnosis is uncertain—particularly in CRMS/CFSPID. The new Journal of Clinical Medicine (Aug 26, 2025) pilot confirms that a standardized, low-volume Ussing setup can differentiate cohorts on physiology (not just genotype), strengthening the case for broader ICM adoption in clinical and translational programs.
Source: PubMed 40066329
The reproducible ICM blueprint (directly traceable to the paper)
Hardware & environment
-
Chamber: EasyMount Low-Volume Ussing System P2400 (Physiologic Instruments)
-
Slider: P2407B (for small rectal biopsies)
-
Temperature: pre-incubate chambers at 37 °C
-
Voltage clamp: measure PD, Isc, and Rt per SOP v2.7
Sample handling & timing
-
Rectal biopsy collection: ≥3–4 fragments per subject with 2.3 mm disposable forceps
-
Transport: immediately into ice-cold PBS
-
Mounting window: 10–20 min from biopsy start to experiment start
-
Baseline: after ~5 min pre-incubation, record PD_basal, Isc_basal, Rt_basal
-
Viability: include responses only if tissue is intact and Rt > 10 Ω·cm²
Stimulus sequence (mucosal/serosal as specified)
-
Amiloride (100 µM) → IBMX (10 µM) + forskolin (100 µM) → carbachol (100 µM) → genistein (50 µM) → DIDS (200 µM) → histamine (500 µM)
-
Compute cumulative secretion (e.g., carbachol + IBMX/forskolin + histamine) for group comparisons.
Results the setup can resolve
-
Cumulative ΔIsc (carbachol + IBMX/forskolin + histamine):
-
CF: 15.32 ± 15.47 µA/cm²
-
CRMS/CFSPID: 86.84 ± 37.84 µA/cm² (p < 0.001 vs CF)
-
Healthy controls: 80.16 ± 48.54 µA/cm² (p = 0.005 vs CF)
→ Practical implication: power future studies around cumulative responses; these combinations are highly discriminative for CF vs non-CF.
-
Equipment transparency (and where to find it)
The study explicitly names the EasyMount P2400 Ussing system and P2407B slider. For labs building capacity, this low-volume architecture helps conserve reagents and stabilizes small tissues—advantages when working with pediatric biopsies or high-cost modulators.
Implementation notes for new ICM programs
-
Adopt ECFS DNWG SOP v2.7 and stick to the paper’s timing windows; both are critical for reproducibility across sites.
-
Standardize viability thresholds (e.g., Rt > 10 Ω·cm²) and reject runs that miss QC—this was essential to the authors’ dataset quality.
-
Report make/model of chambers and sliders in the Methods—this paper sets a high bar for equipment disclosure and enables meta-analyses downstream.
Full credit to the authors
Postek M., Zybert K., Wozniacki L., Woynarowski M., Sands D. Pilot Evaluation of Intestinal Current Measurement in Cystic Fibrosis and CRMS/CFSPID Patients in Poland. Journal of Clinical Medicine. 2025;14(17):6020. Published: Aug 26, 2025. DOI: 10.3390/jcm14176020. We thank the team at the Cystic Fibrosis Center in Dziekanów Leśny for openly detailing their protocol and hardware choices. MDPI